What Makes Soil Corrosive?
- Home
- What makes soil corrosive
Soil contains water and dissolved minerals responsible for damaging an
underground tank. All soil contains water. Even desert sand contains
underground
moisture.Let's assume that you are responsible for deciding whether or not
corrosion will affect a new tank to be installed at a new home in the
desert.
First, let’s begin with the questions:
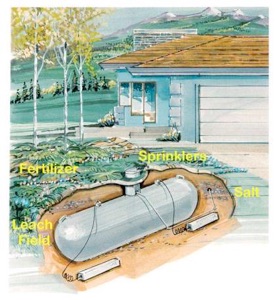
Will it ever rain or snow there? Will the new owner ever plant grass,
flowers,
shrubs or trees over or near the tank? Will ground moisture condense on the
outside surface of the tank? Will water condense out of thin air on the
surface
of a cold container? Will the grass, flowers or shrubs over the tank be
fertilized? Will the tank ever be near a sidewalk or down gradient from a
driveway? Will the owner ever throw salt or calcium chloride on the pavement
to
melt snow and ice? Will magnesium chloride ever be used to control road dust
nearby? Will the tank ever be located near or downhill from livestock where
salt
and urea from animal waste will leach into the earth? Does the irrigation
water
contain salt or alkali salts? If a tank is set in a hole blasted from solid
rock, does the rock contain pyrites, graphite or acidic rocks that will be
fractured and exposed to the oxidizing effect of air and water?
Pyrite (iron sulfide) exists in black shale, coal, limestone, metamorphic
rocks,
in mining districts and in oxygen deficient mud. It oxidizes into iron oxide
which appears as rust staining and a mild sulfuric acid. Graphite (carbon)
is
corrosive to iron as previously mentioned. It is found in metamorphic rocks
of
New England.
If the tank is backfilled with sand or gravel from a sand and gravel
company,
will the sand contain minerals mined from river deposits not representative
of
the site in which the tank is buried? Will the tank be downhill from a
septic
tank leach field? Whatever flows into the septic tank will be deposited in
the
ground below the drain field. Will the soil contain gypsum? Anaerobic
bacteria
eat gypsum (calcium sulfate) and produce sulfide wastes that in turn becomes
sulfuric acid. Will the tank be set in shale or clay? Will groundwater be
present? Will the tank be set on undisturbed earth and backfilled with loose
backfill?
Due to the difference in the oxygen content of the soil around the tank
compared
to the less oxygen in the undisturbed earth under the tank, corrosive
conditions
will exist under the tank. If any of the answers to the above questions are
"yes" then the tank will be corroded.